-
 chevron_right
chevron_right
Elon Musk’s Neuralink puts brain chip in first human amid federal scrutiny
news.movim.eu / ArsTechnica · Tuesday, 30 January - 16:20
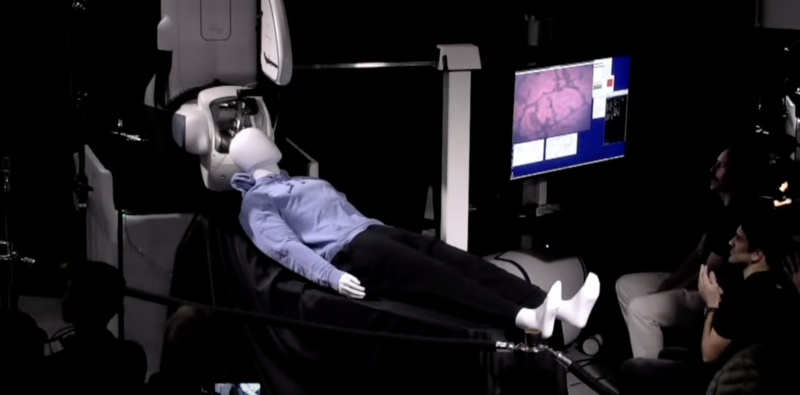
Enlarge / An on-stage demo of the surgical robot. That could be you. (credit: Neuralink )
Billionaire Elon Musk posted on social media late Monday that his brain-computer interface company, Neuralink, implanted an experimental device into the brain of a human for the first time Sunday.
According to Musk's posts , which appeared on the platform formerly known as Twitter, the recipient is "recovering well," and the results in the first 24 hours show "promising neuron spike detection." Neuralink implanted the person with a device called Telepathy, which is intended to allow users to control devices, such as phones and computers, only by thinking.
The implantation comes around eight months after the company announced that the Food and Drug Administration had finally granted a long-sought approval to begin its first human trial. Recruitment for the trial began in September. Musk had claimed to be nearing trials starting as early as 2020, but the FDA reportedly denied approval in 2022 , citing a list of dozens of "deficiencies" and safety concerns that Neuralink had yet to address.


