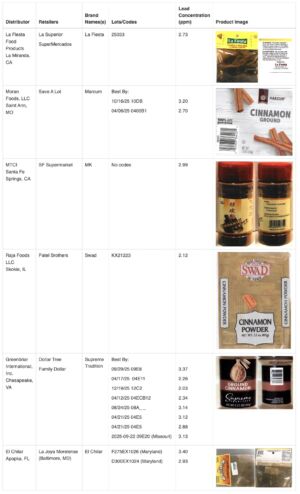
-
 chevron_right
chevron_right
US drug shortages reach record high with 323 meds now in short supply
news.movim.eu / ArsTechnica · Friday, 12 April - 22:20
Enlarge / Takeda Pharmaceutical Co. Adderall XR brand medication arranged at a pharmacy in Provo, Utah, in November 2023. (credit: Getty | George Frey )
Drug shortages in the US have reached an all-time high, with 323 active and ongoing shortages already tallied this year, according to data collected by the American Society of Health-System Pharmacists (ASHP).
The current drug shortage total surpasses the previous record of 320, set in 2014, and is the highest recorded since ASHP began tracking shortages in 2001.
"All drug classes are vulnerable to shortages," ASHP CEO Paul Abramowitz said in a statement Thursday. "Some of the most worrying shortages involve generic sterile injectable medications, including cancer chemotherapy drugs and emergency medications stored in hospital crash carts and procedural areas. Ongoing national shortages of therapies for attention-deficit/hyperactivity disorder [ADHD] also remain a serious challenge for clinicians and patients."