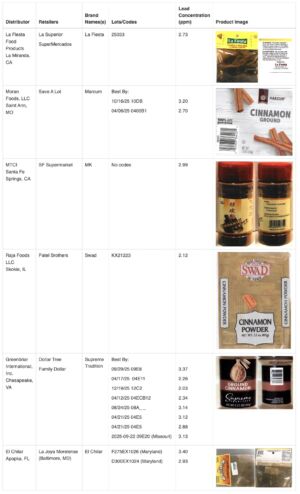
-
 chevron_right
chevron_right
Deadly morel mushroom outbreak highlights big gaps in fungi knowledge
news.movim.eu / ArsTechnica · Friday, 15 March - 16:51 · 1 minute

Enlarge / Mature morel mushrooms in a greenhouse at an agriculture garden in Zhenbeibu Town of Xixia District of Yinchuan, northwest China's Ningxia Hui Autonomous Region. (credit: Getty | Xinhua/Wang Peng )
True morel mushrooms are widely considered a prized delicacy, often pricey and surely safe to eat. But these spongey, earthy forest gems have a mysterious dark side—one that, on occasion, can turn deadly, highlighting just how little we know about morels and fungi generally.
On Thursday, Montana health officials published an outbreak analysis of poisonings linked to the honeycombed fungi in March and April of last year. The outbreak sickened 51 people who ate at the same restaurant, sending four to the emergency department. Three were hospitalized and two died. Though the health officials didn't name the restaurant in their report, state and local health departments at the time identified it as Dave’s Sushi in Bozeman . The report is published in the Centers for Disease Control and Prevention's Morbidity and Mortality Weekly Report.
The outbreak coincided with the sushi restaurant introducing a new item: a "special sushi roll" that contained salmon and morel mushrooms. The morels were a new menu ingredient for Dave's. They were served two ways: On April 8, the morels were served partially cooked, with a hot, boiled sauce poured over the raw mushrooms and left to marinate for 75 minutes; and on April 17, they were served uncooked and cold-marinated.